Six ways to utilize real-world evidence
Real-world evidence is gaining interest – and fast. The phenomenon is global. The growth of the field is evident in the increasing number of data requests and scientific articles. Everyone talks about real-world evidence but how can it be utilized?

My previous post discussed what real-world data, RWD, and real-world evidence, RWE, is. Next we will look deeper into the different uses for real-world data.
Real-world evidence has a wide range of different uses within the scope of healthcare and drug development. At the minimum, an RWE study produces a scientific article that accumulates knowledge in the scientific community. A well-designed RWE study, however, generates outcomes that can be widely used in different contexts.
An RWE study is usually initiated with a specific use for results in mind – such as applying for reimbursement. However, executing RWE studies takes time and often other important but unplanned uses arise during the process.
Real-world data is utilized by the pharmaceutical industry, scientific community, authorities, payers, patient associations, decision-makers, registry-holders, physicians, and service providers. The final beneficiary, however, should always be the patients.
This blog post discusses six different uses for real-world data in the secondary use context. Each of the six areas consist of multiple different applications. This list is not all inclusive since new applications of RWE are constantly discovered.
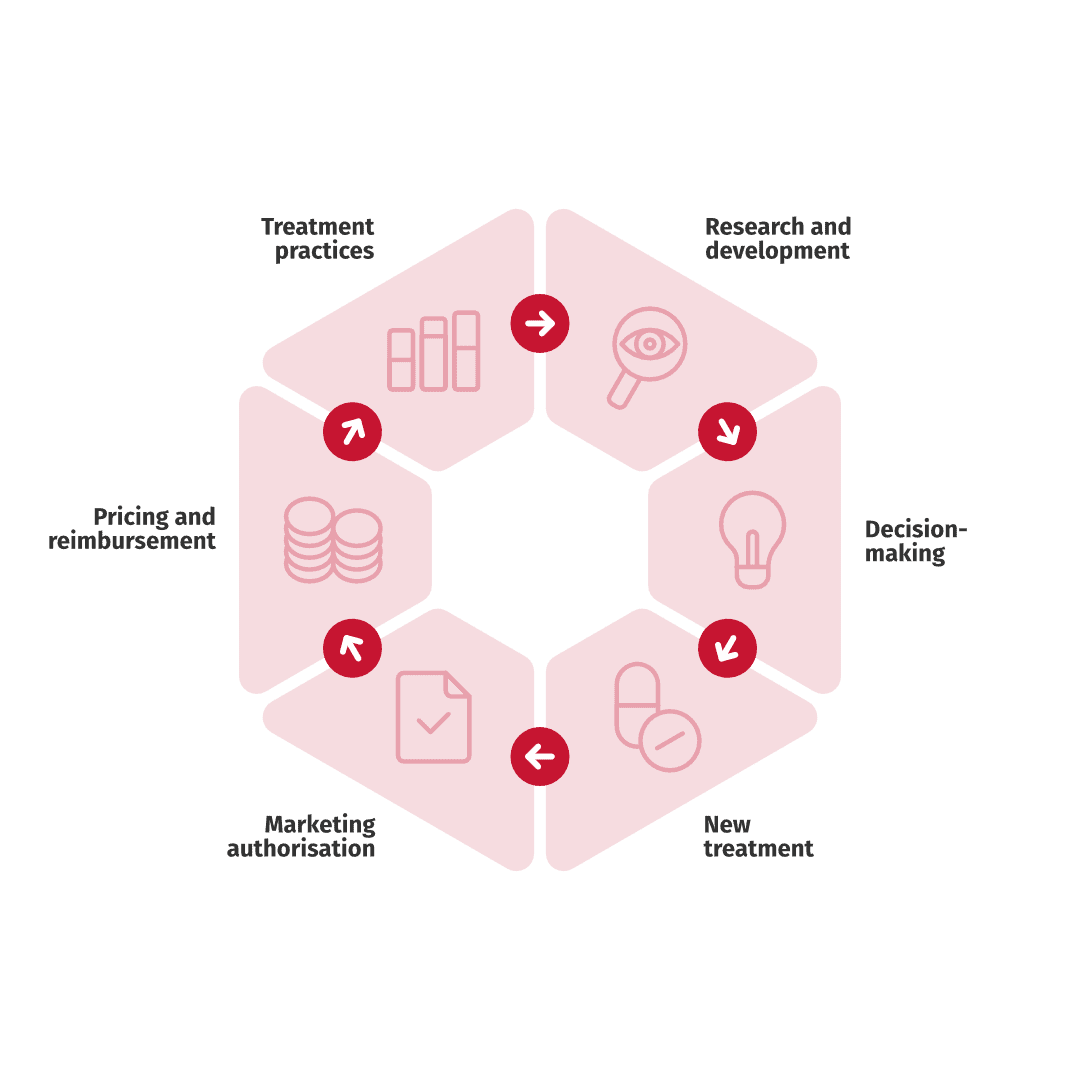
6 uses for real-world data in the secondary use context:
1. Research and development
Real-world data can be utilized in several phases of a medicine’s life-cycle. When development of a new drug molecule begins, existing real-world evidence can be examined from scientific publications. RWE can provide base information to conventional clinical trials, supplement the study data, or even replace the clinical trial altogether. RWE studies are a strong contender compared to clinical trials due to their larger numbered and less delimited study populations. In RWE studies the efficiency of a medicine can be examined on a population level and tracked throughout an extented period.
COVID-19 has affected the treatment of patients which has led, in some cases, to conducting an RWE study instead of a clinical trial.
Biobanks have an important role in research and development as well. New data can be developed by analyzing patients’ samples for new disease markers on top of the existing biobank data.
2. Decision-making
The authority and payer decisions regarding the healthcare system, doctors or specific patient populations aim at cost-effective operations and rational use of medicines. Real-world data can support data-driven decision-making.
The COVID-19 situation increased the demand for real-world data, as healthcare needed to consider their use of resources even more rigorously. Registry data could, for example be utilized in determining the vaccination order for risk groups in a pandemic situation. Furthermore, real-world data can be used to map out risk groups by characterizing risk factors for severe COVID-19 disease symptoms.
3. New treatment into clinical practice
Information on benefits, harms, costs and cost-efficiency is needed before a new treatment is taken into clinical practice. This information can be obtained from a RWE study.
Information on the target patient population can be gathered before the use of a new treatment is initiated. Sometimes the size of the patient population is difficult to evaluate, even for the physicians operating in the field. Real-world data provides trustworthy information on the size of the patient group.
A Registry-based control group for a clinical trial may be formed based on the characteristics of the actual study population using registers. Unlike in the traditional clinical trials, real-world data derived control subjects are not physically involved in the study.
4. Marketing authorization
Real-world evidence can be used to demonstrate the costs and efficacy of the new treatment compared to the traditional treatment option. This information can be used to support a marketing authorization application.
Authorities are especially interested in collecting information on new and innovative treatments, partly because of their costs and partly due to lack of existing evidence on the treatments. These treatments include innovations such as cell- and gene therapies, e.g. CAR-T therapies and CRISPR.
5. Pricing and reimbursement of a medicine
A medicine’s pricing and reimbursement application can be supported using real-world evidence. The costs of traditional treatment and the new treatment can be compared in specific patient groups. Furthermore, real-world data can describe the superiority of the new treatment.
Once the treatment is accepted for clinical use, evidence of its efficiency can be gathered – and in many cases is required by authorities – to re-assess recommendations and reimbursement decisions.
6. Treatment practices
Once the treatment is used in clinical practice, the usage can be examined using registry data. Such examinations may develop treatment recommendations and pathways or provide a new indication for a medicine. Real-world data shows if the dosage in real-world use is similar to what clinical trials indicated or national recommendations suggested. Also, it reveals the actual cost of treatment to the society. A real-world study assesses the efficacy and safety of the treatment .
Conclusions
There are many different uses for real-world data and evidence and new applications are constantly developed. Authorities have voiced their desire to create real-world evidence to support decision-making. The U.S. FDA started a strategic program regarding the use of RWE in determining new indications for medicines, and cell- and gene therapies. European authorities, such as EMA, wish that RWE would be used increasingly to support decision-making. However, the use of RWE is novel and the list of different users and uses of RWE data is currently developing.
There are no established practices in the use of RWE , but we are on our way to establish them. The most effective ways of utilizing RWE studies will be discovered as our experience accumulates and we work together.

